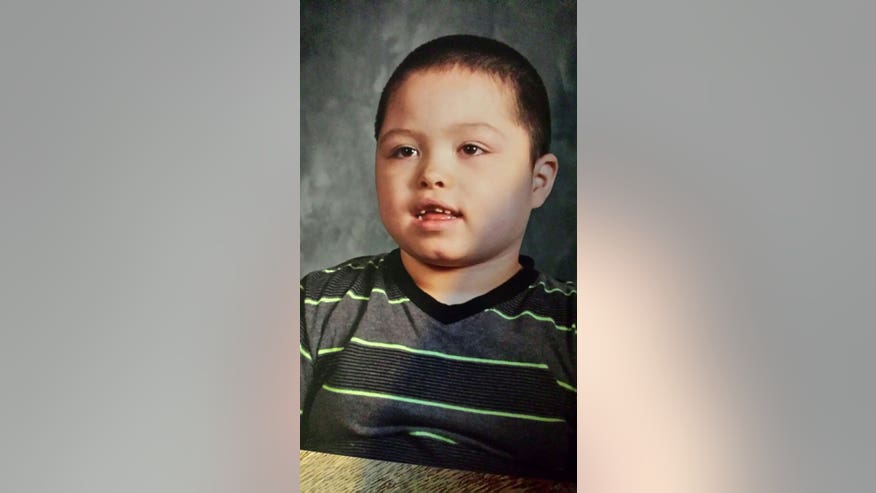
 Six-year-old Izaiah Ruiz has countless seizures a day due to a rare form of pediatric epilepsy. Now, a marijuana-derived drug may help him— and his family— avoid the debilitating attacks.
Six-year-old Izaiah Ruiz has countless seizures a day due to a rare form of pediatric epilepsy. Now, a marijuana-derived drug may help him— and his family— avoid the debilitating attacks.
Izaiah had his first seizure when he was just 2 months old and was diagnosed with Dravet syndrome, a condition that begins in infancy, five months later. The disease, also called Severe Myoclonic Epilepsy of Infancy (SMEI), is intractable, meaning it does not respond to any currently available treatment. While 60 percent of epilepsy cases do respond, 40 percent, including those linked to Dravet syndrome, do not.
When Izaiah has a tonic clonic seizure— the type with muscle spasms that most people think of when they hear the word “seizure”— his grandmother, Lori Fountain, follows a military-like procedure to calm and monitor him.
“Immediately, it’s getting on the floor and laying him down on his side,” Fountain, of Conroe, Tex., told FoxNews.com. “I grab the oxygen and put the oxygen on him. I want the airflow to keep going to the brain and other parts— because when you have a seizure, everything shuts down in your body. You also quit breathing.”
“I grab my phone and I look at the time, and I keep up with the time and I watch him,” Fountain continued, rattling off the directions from a well-rehearsed memory. “Then he will go into the shaking part, and I watch and put the (heart) monitor on him.”
When the seizures last longer than four minutes, Fountain administers Diastat, a rectal medication for cluster seizures. She has the paramedics, who’ve known Izaiah since he was a baby, on speed dial.
Dravet syndrome and Epidiolex
“Most children with Dravet syndrome are having hundreds of seizures a day,” Angus Wilfong, a pediatric neurologist at Texas Children’s Hospital, told FoxNews.com. “Some are subject to status epilepticus, and that’s a life-threatening condition.”
Wilfong is the principal investigator for the trial of a new drug that may help treat Dravet syndrome, which is genetic and currently has no cure. The treatment, called Epidiolex, has transformed the lives of patients with Dravet syndrome without eliciting unusual side effects, Wilfong said.
Izaiah was part of the double-blind dosage-range trial, and for the month of December his grandmother administered the oral liquid, which was created by GW Pharmaceuticals, to him twice a day. Epidiolex reduced his tonic clonic seizures from about four a month to only two, said Fountain, who noted that he still had small seizures daily.
For only the third time in his life, Izaiah was able to spend Christmas at home and not at the hospital.
The controversy over CBD
While Epidiolex holds promise for families like Fountain’s, the drug is controversial due to its makeup: It is comprised of purified CBD, one of at least 80 known molecules of cannabis, or marijuana. Wilfong said there’s no scientific evidence from a formal clinical trial that proves marijuana can effectively treat epilepsy. However, scientists have observed that it can have an effect. They believe that CBD somehow calms the brain activity that causes seizures.
CBD made national headlines in summer 2014 when a Colorado girl, who was also suffering from Dravet syndrome, saw her seizures decrease from hundreds daily to just two or three a month after taking CBD. The medicine has been named “Charlotte’s Web” after the little girl, Charlotte Figi. The medication now reportedly has a waitlist.
Unlike Charlotte’s Web, Epidiolex has no traces of tetrahydrocannabinol (THC), the compound believed to be responsible for marijuana’s psychotropic effects, Wilfong said.
“That’s what differentiates Epidiolex from Charlotte’s Web or from hemp oil or other extracts of the marijuana plant that are available in places like Colorado, where medical marijuana is approved,” Wilfong said. “They’re not using pharmaceutical-grade marijuana. You’re never really sure what you’re getting when you use an extract versus something that’s pharmaceutical.”
Both Charlotte’s Web and Epidiolex have been shown to reduce but not eliminate seizures, Wilfong noted.
According to many medical marijuana advocates, CBD has the potential to fill a treatment void for Dravet syndrome patients. Current treatment options for the condition include: taking one or more of the 30 epilepsy drugs approved by the U.S. Food and Drug Administration (FDA); undergoing brain surgery; going on a high-fat, low-carb Ketogenic diet; or receiving specialized nerve stimulation.
“Despite all of that, these children are still having dozens of seizures a day,” Wilfong said. “That’s why if there’s a signal that something like Epidiolex can help these children, we need to figure it out, and get this treatment available to them to help them with this catastrophic illness.”
Moving forward: Epidolex clinical trials
The trial Izaiah participated in, part A, was conducted to determine the dosage range for the following two clinical trials. The FDA requires two efficacy trials in addition to the dosage-range phase before approving a drug for commercial use.
For part A, a double-blind study, a computer randomly assigned 30 study participants with Dravet syndrome to a low, medium or high dose of Epidiolex, or a placebo. Researchers saw the patients once a week in December. Researchers administered two blood tests to observe how they absorbed and metabolized the medication.
In the next part of the trial, set to launch internationally in the next few months, researchers will use those dosage measurements for 150 new patients. The third and final phase of the trial will study Epidiolex’s effects on another type of pediatric epilepsy: Lennox-Gastaut syndrome.
When Fountain spoke to FoxNews.com, Izaiah was 12 days out from his last dose of Epidiolex. Fountain acknowledged she wasn’t sure if he received the placebo, or what dosage he was given the previous month. But she’s noticed a marked change since he’s stopped taking it: It’s not even halfway through January, and he has already had two tonic clonic seizures in addition to the dozens of tiny seizures he has daily.
Fountain said her wish is for the clinical trials to prove successful so that CBD can be downgraded from its restrictive schedule 1 classification, and then doctors can freely prescribe it to patients like Izaiah.
Based on lack of research, some organizations, such as the American Academy of Pediatrics, do not support medical marijuana for children. But while it is unclear what precise effects marijuana or a marijuana compound may have on children developmentally, families like Izaiah’s say they are running out of options.
“Do your research, and then you can spend a day in my shoes on a day-to-day basis,” Fountain said, “and you would do anything to improve your child’s quality of life.”
“I don’t want to get him high,” she added. “That’s the bad part: [Marijuana] has such a bad stigma that there are people out there who are not willing to open up their eyes.”
Any number of things can set off one of Izaiah’s seizures— if he’s too hot, too cold, stressed, or if he falls out of his everyday routine. While the condition isn’t deadly, Wilfong said, more severe and recurrent seizures prompted by Dravet syndrome increase the chance of sudden or unexplained death.
Dravet syndrome is also defined as a catastrophic form of epilepsy, meaning it affects children developmentally. Izaiah is 6 years old physically but only 2 years old learning-wise, Fountain said.
During the trial, for the first time in his life, Izaiah begged Fountain to decorate the Christmas tree, she said. It was also the first time he took an interest in opening his gifts on Christmas Day.
“He would look at the tree and say, ‘lights,’ every day,” Fountain said. “I tried to get him to say, ‘Christmas,’ but it’s a big word. The fact that he acknowledged the tree and asked me to turn the lights on was big.”
In March, Fountain expects to get access to the open label of Epidiolex with a prescribed dosage for Izaiah. The medication will be available for part A participants when the second part of the trial begins.
Wilfong noted that most clinical research trials for medications seeking FDA approval have an open-label extension phase, held after the initial study period, that participants can voluntarily join. Data is still collected during this time, though in a less rigorous manner, and patients receive the medication for free if they continue to meet inclusion criteria. When the FDA approves any given product, the product is then offered commercially. Open-label phases of trails can last months to several years, Wilfong said.
Until the open-label treatment arrives in her hands come spring, Fountain is holding onto hope.
“That’s what I’m hoping for: that [if] we get him on the right dose, more of him will come out—that maybe if he has a seizure, he won’t lose his words,” she said. “We know he has a lot in there; we just need more of it to come out.”
Melinda Carstensen - Fox News